Research and development of atmospheric pressure sampling ionization method
It is well known that in the cells that make up our bodies, a wide variety of molecules with different molecular weights and chemical properties, such as lipids, metabolites, and proteins, interact with each other to carry out the chemical reactions necessary for biological activities. Imaging techniques to study the distribution of complex chemical species in biological tissues in which diverse cellular networks are formed are important to understand the details of disease states and to make diagnoses.
It is possible to obtain information on multiple molecular ions in a sample as a mass spectrum by extracting the components contained in a localized region of the sample, converting them to gas-phase ions, and introducing them into a mass spectrometer. The distribution of biological molecules can also be visualized from the intensity and the positional information of the individual ions. Imaging technology of various components in biological tissues/cells is expected to be applied to the biomedical research field.
We have developed a unique extraction ionization method “t-SPESI (tapping-mode scanning probe electrospray ionization)” using an oscillating capillary probe (hereinafter called “probe”) and a very small amount of solvent.
Rapid Communications in Mass Spectrometry, 26 (2012), 2725-32.
Analyst, 139 (2014), 2336-41.
Journal of Mass Spectrometry, 50 (2015), 1157-1162.
Mass Spectrometry (Tokyo), 7(2) (2018), S0078.
Analytical Chemistry, 93 (2021), 2263–2272.
Analyst, 148 (2023), 1275-1284.
Analytical and Bioanalytical Chemistry, (2024)
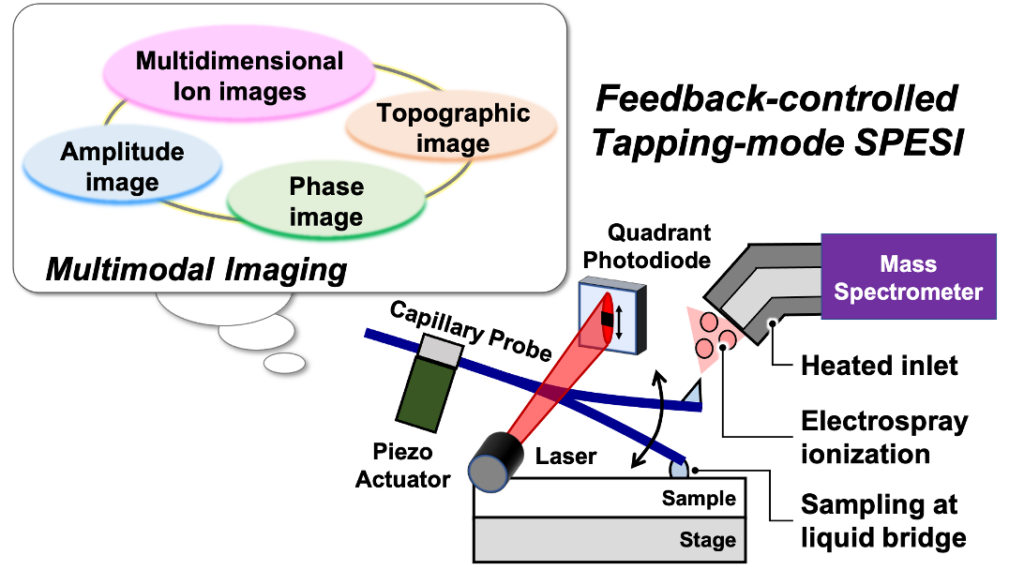
In t-SPESI, molecules contained in a localized region of a sample can be rapidly extracted and ionized by intermittently supplying the sample with picoliters of charged solvent via a rapidly oscillating probe. Ionization is performed by electrospray ionization, which allows biomolecules to be converted to gas-phase ions without structural fragmentation. By scanning the probe in a two-dimensional direction relative to the sample surface, a mass spectrum associated with the sample coordinates can be obtained, thus the distribution of specific molecules within the sample can be visualized.
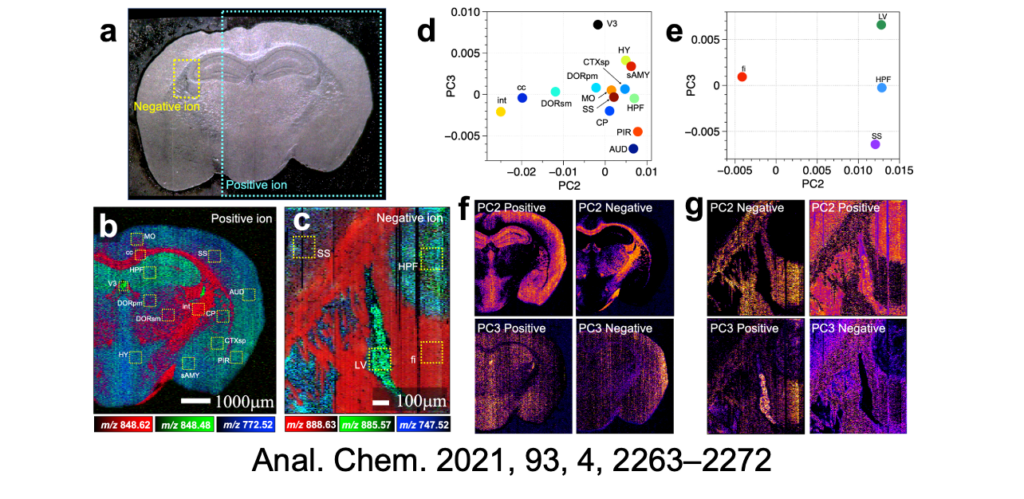
We have integrated the elemental technology of atomic force microscopy, which enables measurements of the topography of a sample at the nanoscale, into t-SPESI to realize a technique for extraction and ionization while measuring the surface topography of the sample in real-time. Using this technology, we were able to image multiple lipids in mouse brain tissue sections with a spatial resolution of 6.5 micrometers and showed that it is possible to classify brain structures based on differences in the mass spectral patterns of multiple lipids.
We are also conducting collaborative research on diseased tissues for medical applications of t-SPESI. Through the joint medical-engineering collaboration at Osaka University, we have succeeded in visualizing the diverse distribution morphology of various lipid groups in human heart disease tissues. In the future, we will continue our research on the physical understanding and control methods of the extraction ionization process, with the aim of achieving high spatial resolution imaging of a single cell.
Development of projection-type imaging mass spectrometry (Mass Microscope)
We are developing a novel projection imaging mass spectrometer (Review of Scientific Instruments, 92 (2021), 053706). A projection-type mass spectrometer simultaneously ionizes a wide area of the sample surface, projects the ion distribution onto a position-sensitive detector, and performs mass analysis at the same time. Currently, we are working with Ueda Laboratory of Graduate School of Frontier Biosciences to develop a single-cell imaging technology.
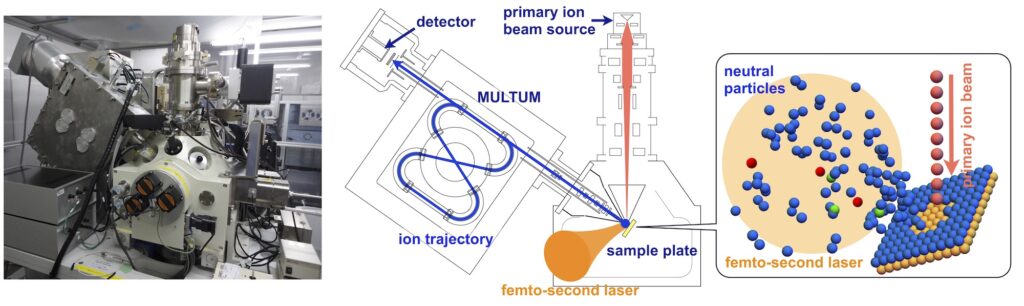
Development of MULTUM-SNMS (Secondary Neutral Mass Spectrometer)
In order to measure the abundance of atoms and molecules in a sample and their isotopic composition with a spatial resolution less than 1 µm, the sample is sputtered by a focused ion beam and then post-ionized using a high-power laser. We are developing a Secondary Neutral Mass Spectrometer for submicron Imaging mass spectrometry (JPS Conf. Proc., 31 (2020), 011065). We are working with the Terada Laboratory of the Department of Earth and Space Science. The equipment is installed at Terada Lab.